Documentation
The Full Package
All systems are supplied with a complete documentation package in line with FDA requirements. A complete documentation package is issued for review and approval within three weeks of order placement.
Documentation You'll Recieve
-
Quality Plan Document
Quality-control inspection procedures will be completed against the activities detailed within the quality plan, with information recorded and retained in the Equipment Data Book. Each activity will be dated and signed by the person responsible, with the client reviewing information and confirming each task has been completed as required. Including Project time plan (milestones) and Quality Assurance & Quality Control.
-
Qualification Master Plan Document
The objective of this protocol is to define the Factory Acceptance Tests and Site Acceptance Tests, including Installation and Operational Qualification requirements and acceptance criteria for the Facility, for installation by the client.
-
Design Qualification Document
The Design Qualification and associated documents are to provide verification that all aspects of the design comply with the User Requirement Specification; including technical specification, design calculations, fan performance curves, motor data sheets, technical specifications for main components and spare parts list.
-
FAT (Factory Acceptance Tests) Document
To confirm the system achieves its functional requirements and meets the required design requirements and quality standards.
-
SAT (Site Acceptance Tests) Document
The objective of this protocol is to define the installation checks and operational test requirements and acceptance criteria for the system.
-
IQ (Installation Qualification) Protocols and Documents
Checks to confirm the system is installed in accordance with the approved specifications and drawings. Calibration of test instruments and system instrumentation. Material test certificates, electrical test certificates, certificates of conformity for main components.
-
OQ (Operational Qualification) Protocols and Documents
Functional checklist; operational tests to confirm: airflow velocities, noise levels, lighting levels, airflow smoke patterns, filter validity testing, airflow quality particle counts.
-
Certification Documents
Include original certification documents, CEE certification and test results.
-
Operations and Maintenance Manuals Documents
Operating procedures, maintenance schedules, corrective maintenance, spare parts identification, operator training, maintenance training.
-
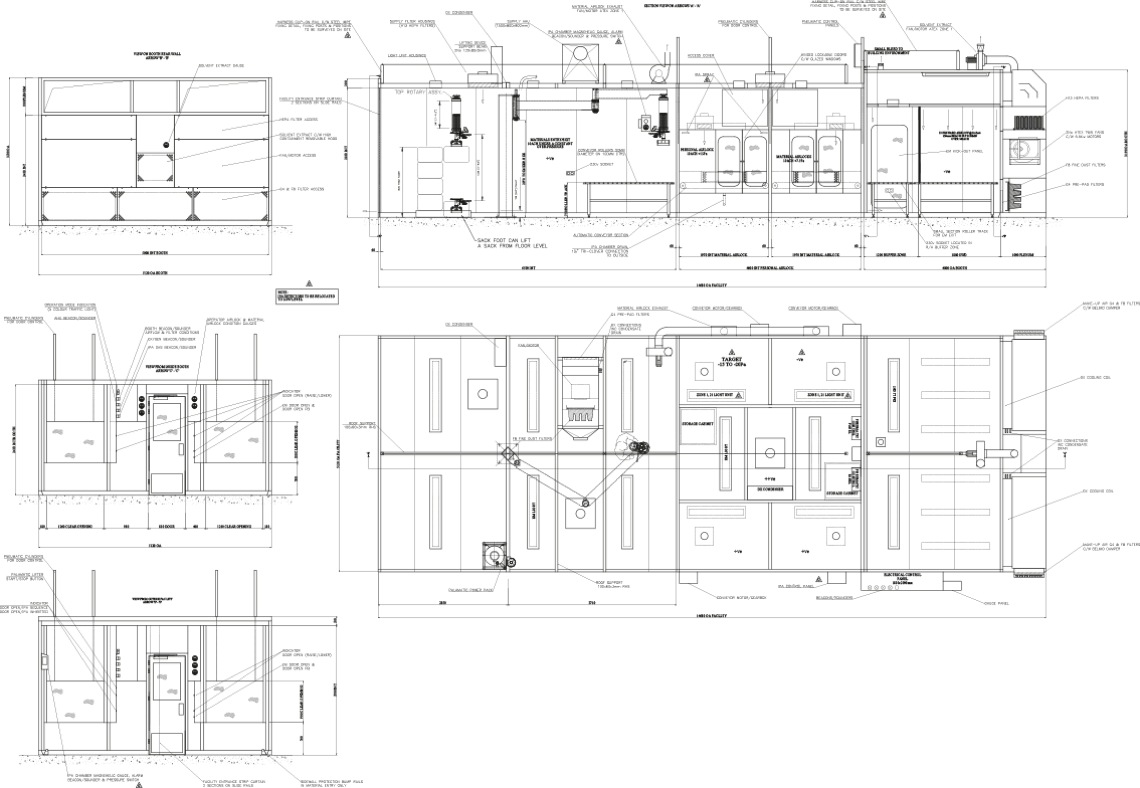
Drawings
General arrangement drawing, piping and instrumentation drawing (PBID), operational sequence diagram, electrical drawings.
Example of a sampling facility drawing